By Ashley O’Neill (Contributor) – Email
Print Edition: September 11, 2013
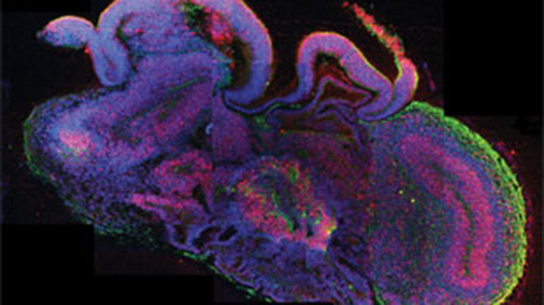
This summer Austrian scientists grew a clump of cells that resembled an embryonic human brain.
The three-dimensional brain model was grown successfully in just under a month by a team at the Institute of Molecular Biotechnology of the Austrian Academy of Science.
With the right concentration of nutrients in a spinning chamber, the human stem cells slowly developed into more complex cerebral organoids. Scientists observed the small balls of white tissue as they quickly grew over the four week period; however, the growths were still no bigger than the size of a pea. Scientists credit the fast growth to the nutrient syrup the stem cells were soaking in, as it helped the cells approach a neural state and offered structural support. The cells also absorbed more nutrients with the help of the spinning bioreactor.
“This demonstrates the enormous self-organizing power of human cells,” Jürgen Knoblich said to The Scientist. “Even the most complex organ—the human brain—can start to form without any micro-manipulation.”
The brain model has the distinct layers and regions found in human brains, such as the prefrontal cortex, occipital lobe, hippocampus, and retinas. Even in the brain’s early development, scientists were able to identify neuron structures and tissues.
The success of growing this organoid opens up the possibility of scientists getting deeper insight into how the brain develops in its earliest stages and any disorders that disturb these first steps. Though the success of this experiment is rather encouraging, the cells are still very simple and underdeveloped compared to those in a mature brain, which will not play a critical role in leading scientists to a better understanding of the neuron network of an adult human brain.
“We’re [just] talking about the very first steps of embryonic brain development, like in the first nine weeks of pregnancy,” Knoblich said.
He also told reporters from Reuters (UK) that the small size of the organoid was not an issue in their research.
“Our system is not optimized for generation of an entire brain and that was not at all our goal. Our major goal was to analyze the development of human brain (tissue) and generate a model system we can use to transfer knowledge from animal models to a human setting,” Knoblich said.
In fact, the mini-brains were used to model microcephaly, a genetic mutation that stunts brain development, resulting in a smaller brain. The team reprogrammed skin cells from a patient affected by microcephaly into stem cells, which they then grew into brain organoids. The gene mutation consequently caused the microcephaly-afflicted brain to grow smaller than the mini-brains grew from normal tissue.
Knoblich and his team were able to identify the reason for the stunted size by watching how these organoids developed over the weeks of study. A procedure like this is not easily duplicated with animal models like mice, because their brains are already much smaller than human ones. However, it is unlikely that using human stem cells will entirely replace animal experiments in the near future.
“We can’t duplicate the elegance with which one can do genetics in animal models, but we might be able to reduce the number of animal experiments, especially when it comes to toxicology or drug testing,” Knoblich told The Scientist.
It is unlikely that scientists would be able to grow larger organoids because cells in larger clumps would require some way to transport nutrients through them. Moreover, the interior of such cell clumps would be starved for nutrition, making it even less plausible.
Once scientists can solve this problem, a reality where such brain models increase our understanding of how the brain develops in its later stages will be closer. Described by Knoblich as a “gigantic step forwards,” this would allow scientists to study the development of other disorders such as autism and schizophrenia.